
 The food industry is constantly evolving and change is coming faster than ever for food manufacturers, distributors and retailers. Among the most impactful changes underway is the implementation of the most comprehensive food safety rules in over 75 years via The Food Safety Modernization Act, commonly known as FSMA.
The food industry is constantly evolving and change is coming faster than ever for food manufacturers, distributors and retailers. Among the most impactful changes underway is the implementation of the most comprehensive food safety rules in over 75 years via The Food Safety Modernization Act, commonly known as FSMA.
Literally more than five years in the making, the final versions of three of these regulations were released in the fall of 2015, following the release of two others a few months prior comprising thousands of pages of the Federal Register. Two more rules are due to be released in the final version in mid-2016, completing the process.
The regulations cover areas including produce safety, preventive controls for human and animal food, verification of foreign suppliers, third-party certification protocols, intentional adulteration and sanitary transport.
But let’s put this in some perspective. Food and Drug Administration (FDA) estimates the cost of the rules already issued will range between $1.46 billion and $1.53 billion, annually. That works out to about $13 per U.S. household annually and according to calculations from the Food Institute, Upper Saddle River, New Jersey-based trade association, are equal to about one-tenth of a percent of all U.S. food sales in 2014. It is not known how much of this might be passed onto consumers, however.
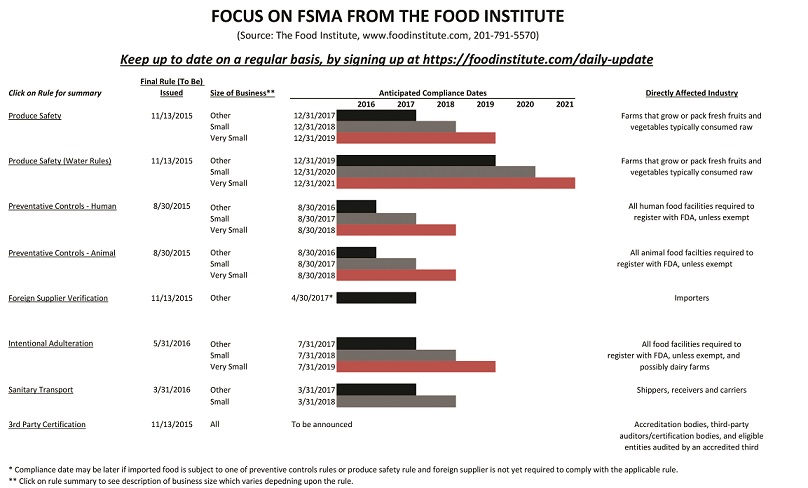
Summaries of the final rules follow, but are not all-inclusive. For more information, go to www.foodinstitute/FSMA or www.FDA.gov.
Produce Safety
The final rule establishes minimum standards for the safe growing, harvesting, packing and holding of fruits and vegetables by farms. It includes the following key provisions:
-
The expanded definition of “farm” is adopted in the Produce Safety rule. The definition of “farm” now includes both “primary production farms” and “secondary activities farms,” the latter being off-farm operations engaged in harvesting, packing or holding raw agricultural commodities. For operations that meet the definition of “farm,” the rule imposes requirements in the following areas: agricultural water quality; biological soil amendments; domesticated and wild animals; worker training and worker health and hygiene; and equipment, tools and buildings.
Foreign Supplier Verification Program (FSVP)
The FSVP requires importers of human food and animal food to develop and implement a Foreign Supplier Verification Program for each food they import into the U.S. and each foreign supplier of that food.
Key provisions of the final rule include the following:
-
The “importer” is the U.S. owner or consignee of a food offered for import.
-
The importer is responsible for the following actions, among others:
-
Conducting a hazard analysis of the imported food
-
Conducting a risk evaluation for the imported food and the foreign supplier
-
Conducting foreign supplier verification activities
-
Taking corrective actions as appropriate
-
Establishing and following written procedures to ensure it imports food only from approved foreign suppliers.
-
-
Foreign supplier verification activities may include: annual onsite audits of the foreign supplier performed by a qualified auditor, sampling and testing of food, review of the foreign supplier’s food safety records, and/or other appropriate activities.
Third-Party Certification
This rule establishes the framework, procedures and requirements for accreditation bodies to be recognized by FDA and for third-party certification bodies to be accredited by accreditation bodies. It also includes requirements for audits and issuance of food safety certifications by accredited third-party certification bodies.
-
Food and facility certifications issued by accredited third-party certification bodies are intended to be used for the following purposes:
-
Food and facility certifications issued by accredited third-party certification bodies can be used by importers to establish eligibility for the Voluntary Qualified Importer Program (VQIP). VQIP offers participating importers expedited review and entry of the foods they import.
-
FDA may make a risk-based determination to require that a food imported or offered for import into the U.S. be accompanied by a certification or other assurance that the food meets the applicable requirements of the FD&C Act. When FDA has determined that a food import is subject to such certification, FDA will require, as a condition of entry, a certification issued either by an accredited third-party certification body or by an agency or representative of the government of the country from which the food originated.
Preventive Controls for Human Food
This rule creates certain new requirements for the production of human food by registered food facilities, and revises previous requirements, in three key ways:
-
First, the final rule modernizes FDA’s long-standing current good manufacturing practice (CGMP) regulations, which govern the manufacturing, processing, packing or holding of human food. It imposes minimum training requirements for all employees involved in manufacturing, processing, packing or holding food (including temporary and seasonal employees). Second, the final rule requires that covered facilities establish and implement hazard analysis and risk-based preventive controls for human food. In general, these requirements apply to establishments that are required to register with FDA as a food “facility.” This portion of the final rule requires registered food facilities to maintain a food safety plan, perform a hazard analysis and institute preventive controls for those hazards, unless an exemption applies. Third, the final rule clarifies the scope of the exemption for farms in FDA’s current food facility registration regulations, and makes corresponding revisions to FDA’s current regulations for the establishment, maintenance and availability of records.
Preventive Controls for Animal Food
The final rule establishes new requirements for the production of animal food by registered food facilities in two ways:
-
First, the final rule creates new CGMP regulations that specifically address the manufacturing, processing, packing and holding of food for animals. These requirements apply to establishments that are required to register with FDA as a food “facility.”
-
Second, the final rule requires covered facilities to establish and implement hazard analysis and risk-based preventive controls for food for animals. As with the CGMPs, these requirements apply to establishments that are required to register with FDA as a food facility. This portion of the rule requires registered animal food facilities to maintain a food safety plan, perform a hazard analysis and institute preventive controls for the control of those hazards, unless an exemption applies.
The final rule also implements the requirements of FSMA for covered facilities to establish and implement a food safety system that includes a hazard analysis and risk-based preventive controls. Specifically, the final rule provides for the following:
-
Covered facilities must establish and implement a food safety system that includes an analysis of hazards and risk-based preventive controls. The rule sets requirements for a written food safety plan that includes:
-
Hazard analysis
-
Preventive controls
-
Oversight and management of preventive controls, including
-
-
Monitoring
-
Corrective actions and corrections
-
Verification.
-
-
A covered facility also would be required to maintain associated records and have a recall plan
-
-
The supply chain program is made more flexible, with separate compliance dates established.
So times certainly are changing to ensure the U.S. food supply remains the safest in the world. Many industry players are already taking steps to implement these new rules and if you are not, it is time to start. The Food Institute is here to help as the implementation process continues.

